RESEARCH INTERESTS
OLD TECHNIQUES, BUT NEW LOOKS AT BIOMEDICAL CHROMATOGRAPHY
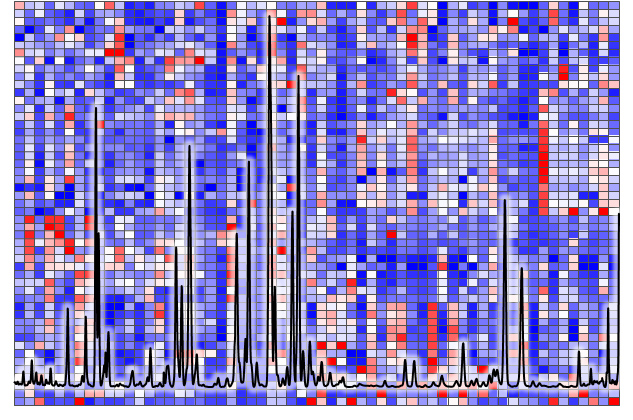
The initial report in 2007 introduced a gas chromatography-based separation method coupled with single-quadrupole mass spectrometric detection for the screening of 72 endogenous steroids. Subsequently, targeted assays for specific steroids, including sterols, progestogens, mineralo-/gluco-corticoids, androgens, and estrogens, have been developed using both gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) techniques, showcasing heightened analytical sensitivity and selectivity. To ensure the reliability of results, these analytical platforms necessitate meticulous sampling and comprehensive purification steps. Moreover, they facilitate the accurate quantification of respective steroid hormones across a range of biological specimens, such as blood (serum/plasma), urine, tissue (snap-frozen/paraffin-embedded), blood spots, saliva, and hair fibers.
ESTABLISHING STEROID PANELS IN TRANSLATIONAL RESEARCH
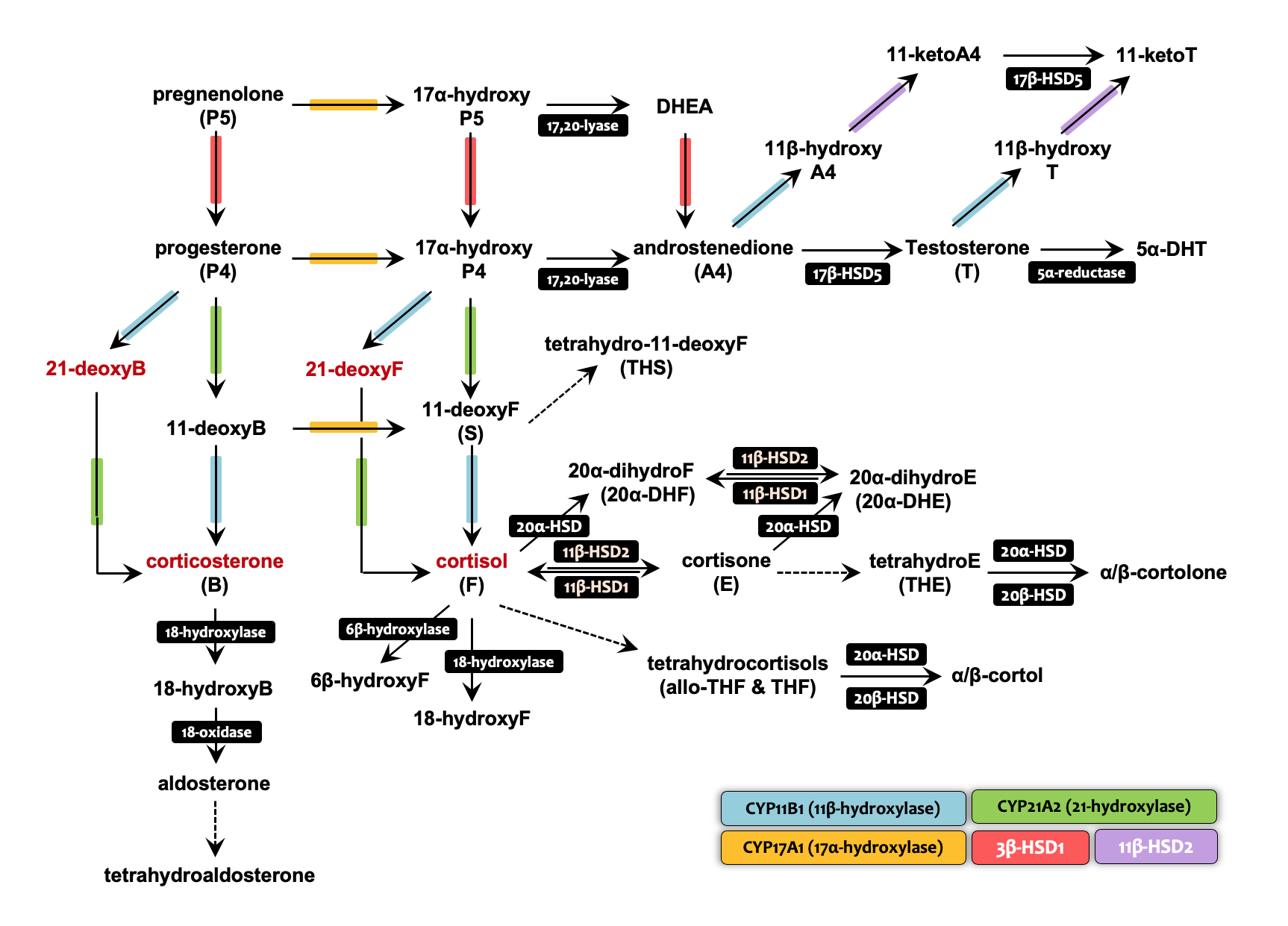
The quantitative assessment of endogenous steroids is integral to understanding metabolic homeostasis across diverse physiological conditions, given their crucial biochemical functions within the endocrine system. Our research is specifically dedicated to unraveling the actions of enzymes associated with steroidogenesis. The measurement of enzyme activity, expressed through the metabolic ratio between precursor and metabolite, presents significant potential as a valuable tool for clinical diagnosis and biomarker discovery using various biological specimens. A distinctive feature of our study is the introduction of the concept of a "metabolic signature", which characterizes endocrine diseases. As an illustrative example, the application of a steroid panel consisting of 16 serum steroids for subtyping adrenal diseases, including adrenal tumors and congenital adrenal hyperplasia, holds promise in facilitating improved patient care and enhancing clinical outcomes through a single analytical run.
UNDERSTANDING ADRENAL AND GONADAL STEROIDOGENESIS
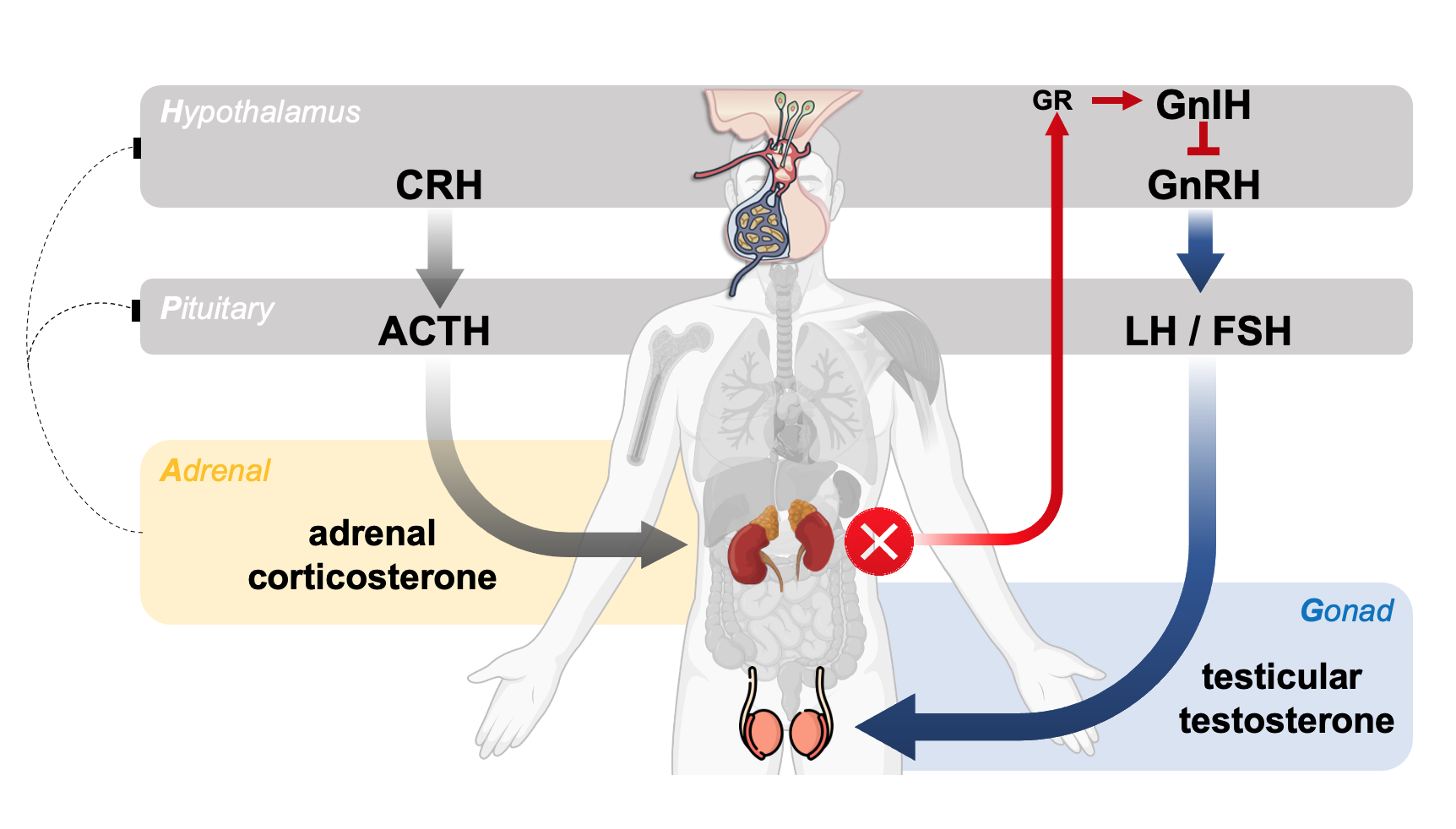
The hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–gonadal (HPG) axes exhibit reciprocal relationships in the regulation of steroidogenesis. The integration of our mass spectrometry-based profiling techniques with molecular endocrinology yields comprehensive physiological insights into both adrenal and gonadal steroidogenesis. In animal studies, both the delta-4 and -5 steroidogenic pathways contributing to testosterone production show heightened activation during prenatal development compared to the postnatal period. Furthermore, androgen-induced suppression of global transcription mediated by Ad4BP/SF-1 implies sexual dimorphic corticosterole levels, potentially influencing sex differences in skeletal muscle. Additionally, the interplay between defective adrenal corticosterone secretion and increased testicular production underscores the crosstalk between the HPA and HPG axes in the regulation of homeostatic steroidogenesis.